

David Trew
Consulting Ltd
Management of Equipment in an ISO/IEC 17025:2017 Accredited Laboratory
Part 2: Equipment Lifecycle Models
1 Introduction
ISO/IEC 17025:2017 General requirements for the Competence of Testing and Calibration Laboratories1 is an international quality assurance scheme for ensuring the quality of testing and calibration operations and competency of those that carry out those activities. The goal of the ISO/IEC 17025 International Standard is to facilitate the global recognition of testing and calibration results by serving as a framework of requirements for individual testing and calibration laboratories to construct a quality management system appropriate to their needs.
The first paper2 in this series discussed the classification of laboratory equipment into different categories. The categories were either based on the use of the equipment or the equipment’s complexity. These are summarised in Table 1.
Table 1: Equipment Classifications
Clause 6.4 of the ISO/IEC 17025 International Standard addresses the requirements for laboratory equipment, including measuring instruments. This Clause requires laboratory measuring instruments to be suitable for purpose and conform to pre-established specifications from when it enters service until it is decommissioned at the end of its useful life. One way of achieving this is to adopt a life cycle management model approach, with milestones at key points corresponding to key events on the instrument’s lifecycle.
The benefits of implementing life cycle management model are:
1. Appropriate specifications for the equipment are established before procurement
2. Equipment is demonstrably suitable for its purpose before it is used to perform any testing or calibrations and throughout its service life.
3. The equipment is calibrated and maintained to ensure it continues to be suitable for its purpose.
4. Evidence is continually created to demonstrate the equipment continues to be suitable for its purpose throughout its service life, and any performance deterioration is quickly detected and corrected.
5. The performance of the equipment is periodically reviewed to detect any long-term trends in performance and to ensure the equipment always conforms to current requirements
6. The performance at the end of the equipment’s life is confirmed to meet established requirements.
7. All equipment is managed in a consistent manner
8. The model is designed to ensure equipment is demonstrably suitable for purpose throughout its entire life
Implementing such a lifecycle into a laboratory’s quality management system requires that the model must be clearly defined and will require appropriate procedures to be established to provide instructions on how to perform the associated management tasks consistently. This paper will discuss the structure of a suitable lifecycle, together with the content of the procedures required to manage it.
2 Equipment Life Cycle
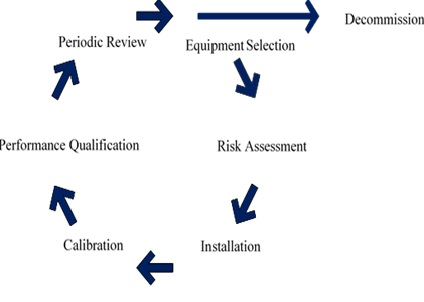
The equipment life cycle shown in Figure 1 is designed to ensure laboratory equipment is suitable for its purpose and continues to provide valid results throughout its entire lifetime.
|
Level
|
Quality Criticality
|
Measuring Equipment
|
Analytical Equipment
|
Software
|
|
1
|
Quality Critical
|
Reference Standards
|
Non–Measurement
|
Infrastructure
|
|
2
|
Quality Non–Critical
|
Non–Adjustable
|
Non–Computerised
|
Firmware
|
|
3
|
Non–Quality
|
Adjustable Non–Computerised
|
Computerised
|
Non–Customised
|
|
4
|
|
Computerised
|
Networked
|
Customised
|
|
5
|
|
Networked
|
Bespoke
|
Bespoke
|
|
6
|
|
Bespoke
|
|
|
Figure: 1 Equipment Qualification Life Cycle
The management lifecycle consists of the following key points:
1. The cycle starts with equipment selection which entails identifying the user, functional and operational requirements, and assessing the supplier.
2. Following the equipment selection, an assessment of the risks associated with a failure of the equipment’s key functions needs to be made. The information obtained from this risk assessment will be used to identify activities required to mitigate those risks.
3. Installation qualification entails ensuring the equipment was delivered as ordered, the location where the equipment will be used is suitable, and the key functions of the equipment work as described by the manufacturer.
4. Calibration entails referencing the signal from the equipment to recognised metrological standards
5. Performance qualification consists of the daily and periodic checks undertaken to provide evidence the equipment is performing correctly.
6. The periodic review entails reviewing the records created during the use of the equipment to detect any trends or deterioration in performance. If the periodic review, or if at any time, a need to upgrade the instrument is identified, the process of selection, risk assessment, installation, and calibration will start again.
7. Decommissioning entails ensuring the equipment is still within calibration since its previous calibration, all the records are accounted for, and the equipment is cleaned and decontaminated.
 PDF
PDF
PDF
PDF


 PDF
PDF
PDF
PDF