Recently, I posted a discussion of the equilibria associated with multi-protonic compounds with overlapping pKas (ΔpKa < 3). This was illustrated using the example of urocanic acid 2. In contrast to compounds with well separated equilibrium constants (ΔpKa > 3), which protonate in a sequential stepwise manner, compound with overlapping equilibrium constants form a multi-protonic system illustration in Scheme 1.
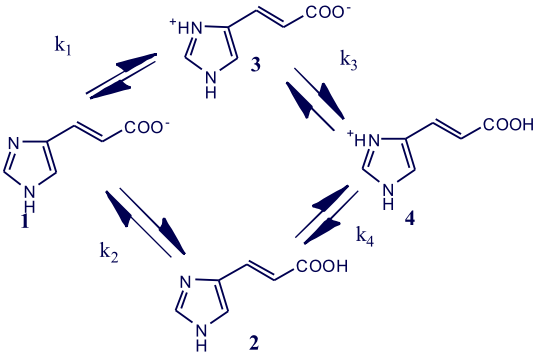
Scheme 1
The species 1 through 4 are known as the microscopic species, and the equilibria k1 through k4 are known as the microscopic equilibria, which are given by:
k1 = [2] k2 = [3] k3 = [4] k4 = [4]
[1][H+] [1][H+] [2][H+] [3][H+]
The macroscopic equilibria of urocanic acid is shown in equation (i):

…..(I)
The macroscopic equilibrium constants of urocanic acid are given by:
K1 = [UrH] K2 = [UrH2+]
[Ur-][H+] [UrH][H+]
These characterise the acid-base equilibria as a whole they do not, however, provide information on the specific protonatable site.
Gradient HPLC of multi-protonic compounds
From the perspective of developing analytical methods, gradient high performance liquid chromatography, multi-protic compounds present a number of challenges. These stem from:
i. The micro-species (1- 4 in Scheme 1), will have different chromatographic characteristics
ii. The relative proportions of the micro-species 1- 4 are dependent on both the local pH and solvent composition of the mobile phase
iii. The equilibrium constants are dependent on composition of the mobile phase
iv. During a gradient HPLC run the solvent composition of the mobile phase is constantly changing, thus the equilibrium constants and the proportions of the micro-species are constantly changing
Therefore, when performing analysis of, or developing HPLC analytical methods for, multi-protic compound using gradient HPLC it is necessary to ensure that the multi-protonic compound is present in only one of its micro-species throughout the entire run. This can be done by ensuring the component mobile phases are sufficiently acidic so that the analyte is maintained in its fully protonated form throughout the gradient profile. Typically this can be achieved by adding between 0.1 and 1.0 % Trifluoroacetic acid to each component of the mobile phase when analysing multi-protonic compounds by gradient HPLC.

 PDF
PDF

